Water is a crucial element to life on Earth. It is essential for our survival and is used in many industries. One of the most important properties of water is its freezing point. In this article, we will discuss the freezing point of water in degrees Celsius and its importance in our daily lives.
What is Freezing Point?

Freezing point is the temperature at which a liquid turns into a solid. In the case of water, it is the temperature at which liquid water turns into ice. This process is also known as solidification.
What is the Freezing Point of Water in Degrees Celsius?
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif)
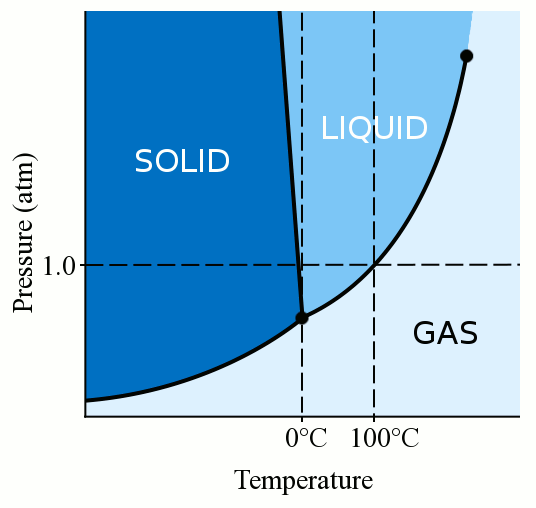
The freezing point of water in degrees Celsius is 0°C. This means that at this temperature, liquid water will turn into ice. However, the freezing point of water can vary based on pressure and impurities in the water.
Why is the Freezing Point of Water Important?

The freezing point of water is important for many reasons. One of the most important is the formation of ice. Ice is essential for many industries such as fishing, transportation, and recreation. In addition, the freezing point of water is important in determining the climate and weather patterns. It is also important for biological processes, such as the survival of aquatic organisms during the winter.
Factors that Affect Freezing Point of Water

The freezing point of water can be affected by several factors. One of the most common is impurities in the water. For example, saltwater has a lower freezing point than pure water. Other factors that can affect the freezing point of water include pressure and dissolved gases.
How to Measure Freezing Point of Water

The freezing point of water can be measured using a thermometer. The thermometer should be placed in the water and the temperature should be monitored as the water is cooled. The temperature at which the water turns into ice is the freezing point of water.
Applications of Freezing Point Depression
Freezing point depression is the process where the freezing point of a liquid is lowered by adding a solute to it. This phenomenon is used in many applications, such as making ice cream. The addition of salt to ice lowers its freezing point, allowing it to melt the ice cream mixture and freeze it at the same time.
Conclusion
The freezing point of water in degrees Celsius is an important property of water. It is essential for many industries and biological processes. The freezing point of water can be affected by several factors such as impurities in the water, pressure, and dissolved gases. Freezing point depression is used in many applications, such as making ice cream. Understanding the freezing point of water is crucial for our daily lives.